Welcome to Neutrium
Neutrium is a knowledge base of engineering topics, centred mainly around chemical engineering design challenges faced by engineers in their daily work. We created Neutrium to bridge the gap between theory and practice. Feel free to ask a question, leave feedback or take a look at one of our in-depth articles.
Dimensionless numbers play an important role in analysing fluid dynamics and heat and mass transfer problems. They provide a method by which complex phenomena can be characterised, often by way of a simple, single number comparison. This article provides a summary of dimensionless numbers and the formulae used to calculate them.
Dimensionless numbers play an important role in analysing fluid dynamics and heat and mass transfer problems. They provide a method by which complex phenomena can be characterised, often by way of a simple, single number comparison. This article provides a brief overview of the derivation and use of dimensionless numbers.

The Sherwood number is a dimensionless number that represents the ratio of convective mass transfer to the rate of diffusive mass transport and is used in the analysis of mass transfer systems such as liquid-liquid extraction. This article describes the Sherwood number and typical formulations.

The Schmidt number is a dimensionless number that describes the ratio of momentum diffusivity to mass diffusivity that is commonly used in analysis of mass transfer systems. This article describes the Schmidt Number and typical formulations.
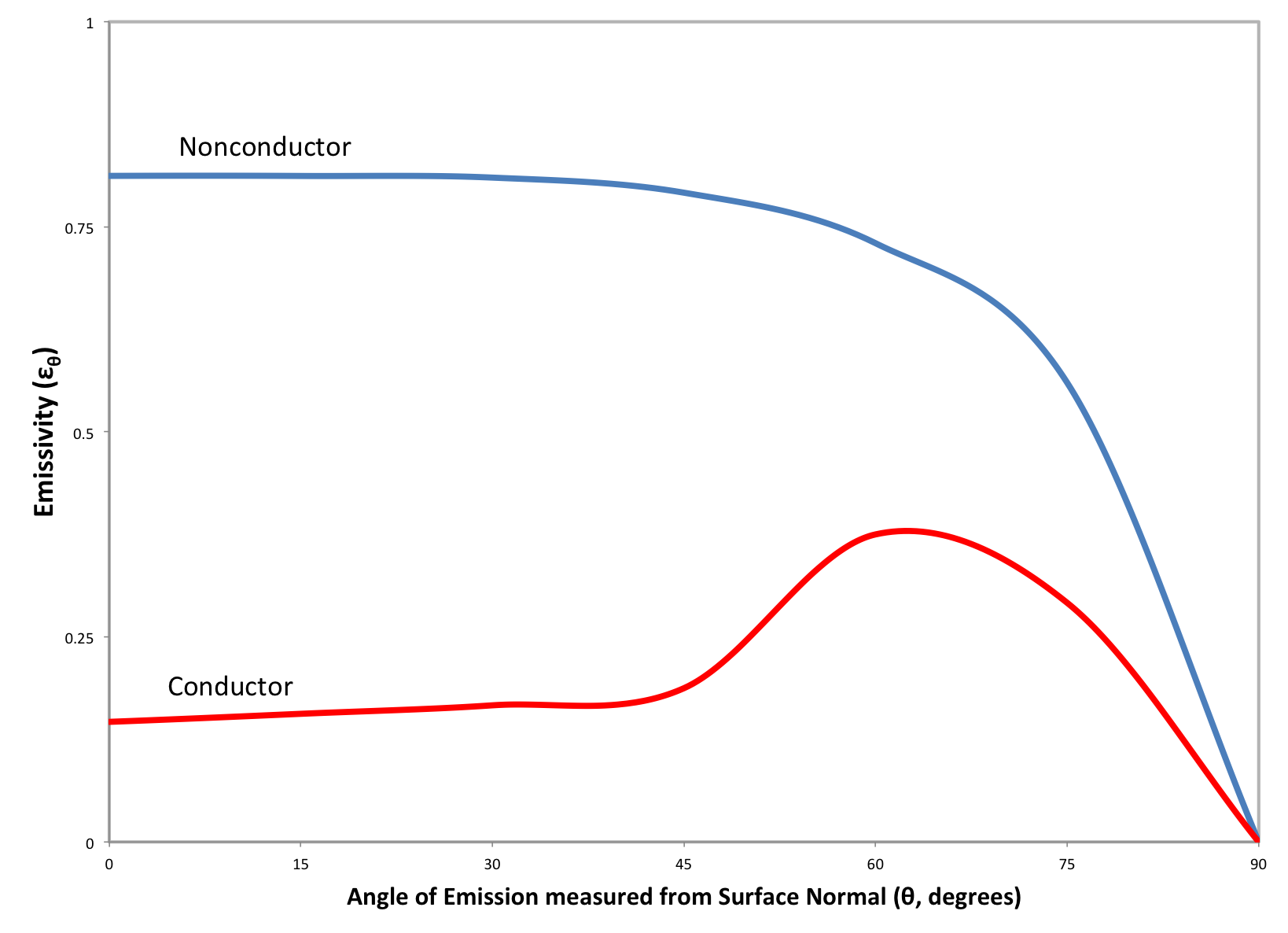
Radiative heat transfer between two or more surfaces can be approximated using the total, normal emissivity. This article provides empirically determined total normal emissivities for a number of materials including metals, metal oxides, common building materials and paints.

Tray efficiency measures the performance of a distillation tray or trays against the maximum theoretical performance. Similarly, a concept called Height Equivalent to Theoretical Plate (HETP) is used to measure the performance in a packed column. This article describes methods of quantifying tray efficiency in distillation tray analysis.

At any given temperature, real materials emit less energy than that of a black body. The effectiveness of a material at emitting energy is represented by a radiative property called emissivity, which is the ratio of the actual energy emitted by the material to that of a black body at the same temperature. This article will provide an overview of the methods available for calculating the spectral, spectral-directional, hemispherical and total hemispherical emissivity for metals.

Relative volatility is a comparative measure of the vapour pressures of components in a liquid mixture. It is commonly used in the design of absorption and separation processes such as distillation as it allows the difficulty of separating components to be quickly assessed.
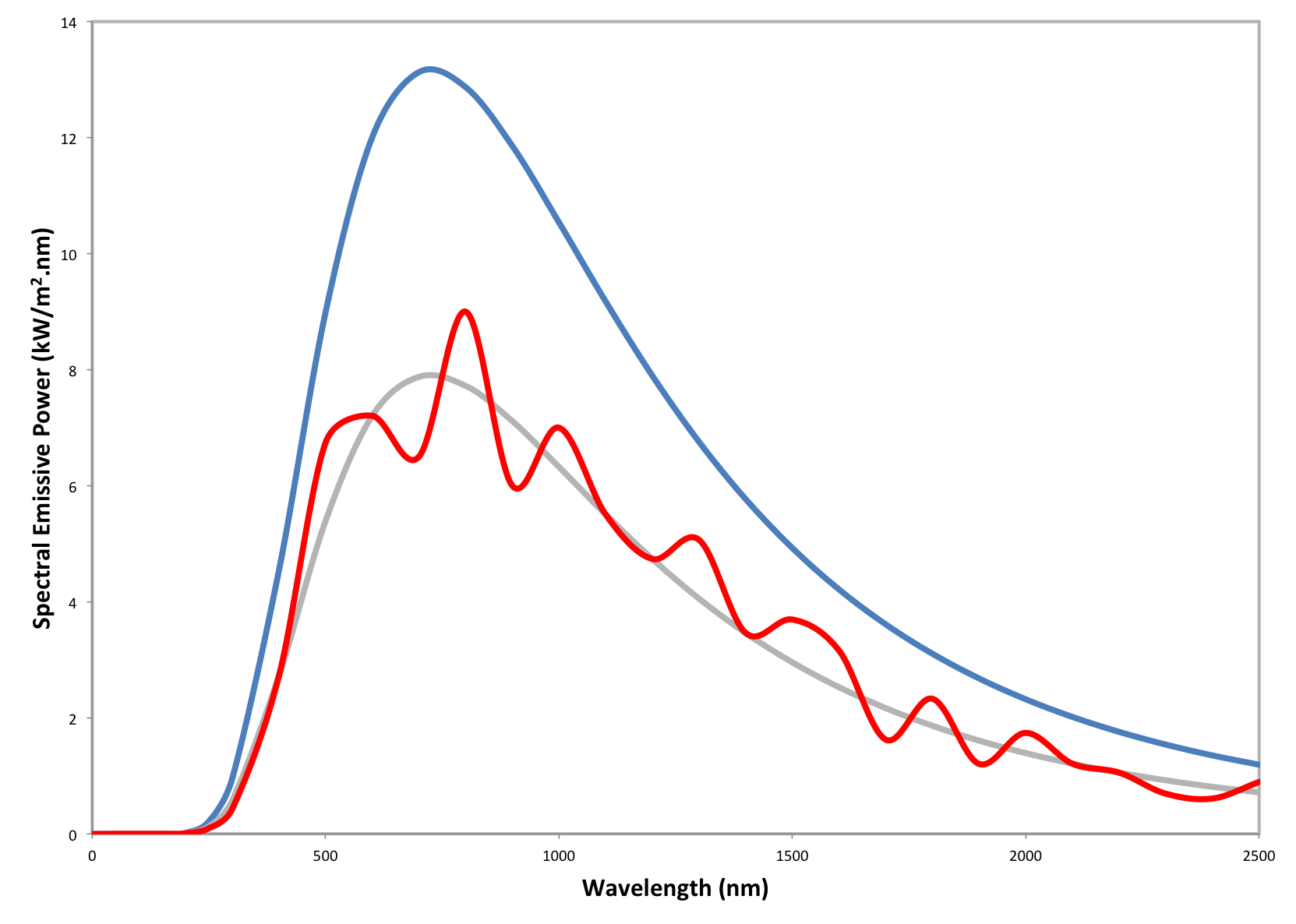
At any given temperature, real materials emit less energy than that of a black body. The effectiveness of a material at emitting energy is represented by a radiative property called the emissivity factor, which is the ratio of the actual energy emission of the material to that of a blackbody at the same temperature. This article will provide an overview of emissivity and its many formulations.

A McCabe-Thiele plot is a simplified tool to assist in understanding distillation. It is a method for calculating the number of theoretical trays required for the distillation of a binary mixture. This article describes how to apply the McCabe-Thiele method.

Distillation is a process by which a liquid mixture is separated into fractions with higher concentrations of certain components by exploiting differences in relative volatility. In industrial settings such as oil refineries and natural gas processing plants this separation process is undertaken using a distillation column. This article describes the basic principles and operation of a distillation column and the equipment and terminology used when discussing distillation.

Henry’s Law describes the relationship between the solubility of a gas in a liquid and the partial pressure of that gas above the liquid surface. A range of experimentally determined Henry’s constants are tabulated and can be used to determine the solubility of various gas species in water.

Raoult’s law gives a method of estimating the composition and pressure of the vapour above a liquid mixture. This article describes the basis of Raoult’s law and provides an example of how to apply it.
Dalton’s law provides a method by which the total pressure of a gas mixture can be calculated using the partial pressures of the component gases of the mixture.

The Wobbe Index is a measure of the interchangeability of fuel gases and their relative ability to deliver energy. It gives an indication of whether a turbine or burner will be able to run on an alternative fuel source without tuning or physical modifications.

The pressure drop or flow rate through a valve or orifice plate is typically calculated using the a flow coefficient, Cv or orifice diameter. This article demonstrates how to convert between these two parameters when performing functions such as selecting a valve with an equivalent pressure drop to a given orifice plate.

The Joule-Thomson Effect describes the change in temperature of a gas as it experiences a rapid change in pressure from passing through a valve, orifice or nozzle. It may represent a safety hazard, or an opportunity depending on the process.
When examining thermodynamic processes some simplifying assumptions may be applied to help describe and analyse a given system. These simplifications can be viewed as ‘ideal’ thermodynamic processes and include adiabatic, isenthalpic, isentropic, isobaric, isochoric, isothermal, isentropic, polytropic and reversible processes. This article provides a brief overview of each process type and suitability to a given thermodynamic system.

Hydrate formation represents a significant risk to process safety as it can result in the plugging of both pipes and instruments. Hydrates typically form in process where light hydrocarbons, water vapor and low temperatures or high pressures are present. This article describes the conditions under which hydrates form, how formation may be prevented and what can be done once hydrates have formed.

Choked flow is a phenomenon that limits the mass flow rate of a compressible fluid flowing through nozzles, orifices and sudden expansions. Generally speaking it is the mass flux after which a further reduction in downstream pressure will not result in an increase in mass flow rate.

The flow of a gas-liquid multiphase system may cause erosion if velocities are high. This article presents an empirical relationship for estimating whether erosion will occur in a system at a certain velocity.

The discharge coefficient is a dimensionless number used to characterise the flow and pressure loss behaviour of nozzles and orifices in fluid systems. Orifices and nozzles are typically used to deliberately reduce pressure, restrict flow or to measure flow rate. This article gives typical values of the discharge coefficient for common orifice and nozzle designs.
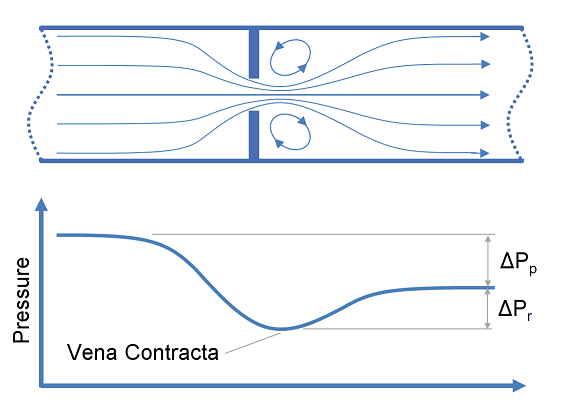
This article provides calculation methods for correlating design, flow rate and pressure loss as a fluid passes through a nozzle or orifice. Nozzles and orifices are often used to deliberately reduce pressure, restrict flow or to measure flow rate.
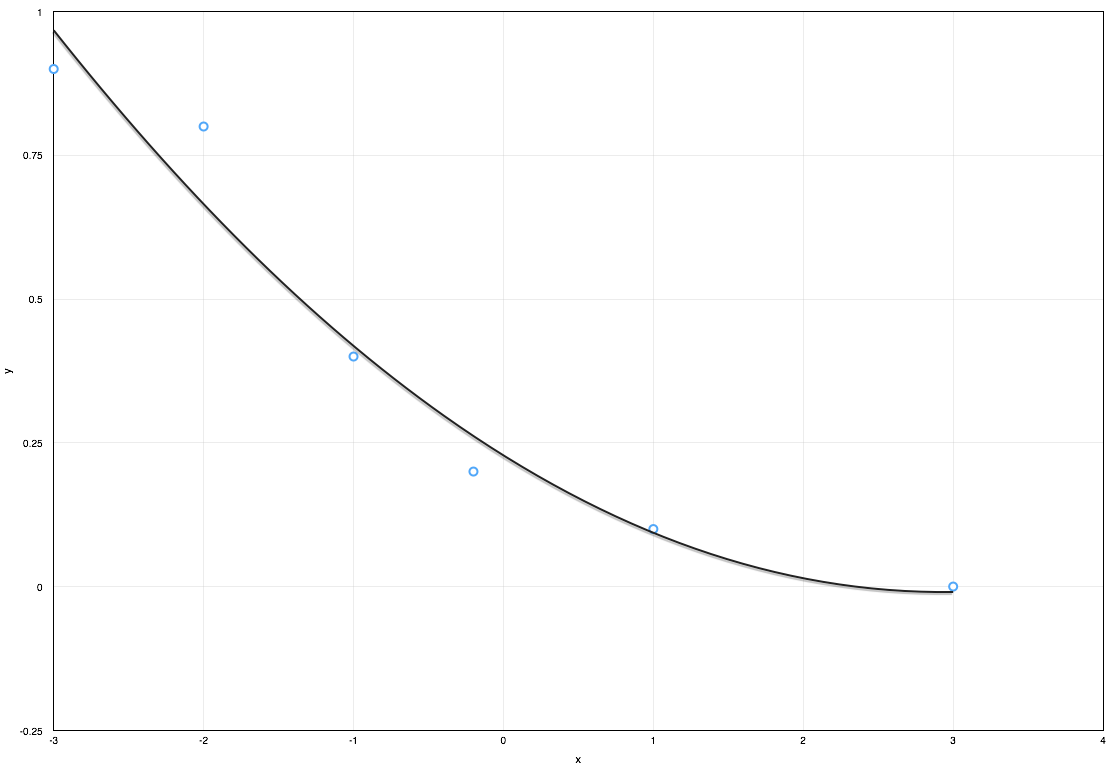
Approximating a dataset using a polynomial equation is useful when conducting engineering calculations as it allows results to be quickly updated when inputs change without the need for manual lookup of the dataset. The most common method to generate a polynomial equation from a given data set is the least squares method. This article demonstrates how to generate a polynomial curve fit using the least squares method.
The Joukowsky equation is a method of determining the surge pressures that will be experienced in a fluid piping system. When a fluid in motion is forced to either stop or change direction suddenly a pressure wave will be generated and propagated through the fluid. This pressure wave is commonly referred to as fluid hammer (also known as water hammer, surge or hydraulic shock) and typically occurs in piping systems when a valve is suddenly closed, isolating the line. The resultant surge pressures are complex to characterise but for simple systems they may be calculated using the Joukowsky equation.